LuluTox Detox Tea Unveiled: Why Lulutox Tea Being Discussed as the Best Herbal Weight Loss Tea Blend for Women to Burn Fat in 2026
LuluTox Detox Tea Unveiled: Why Lulutox Tea Being Discussed as the Best Herbal Weight Loss Tea Blend for Women to Burn Fat in 2026
Discover Lulutox Detox Tea, a herbal blend designed to support digestion, detox balance, and daily wellness. Ingredients, usage, and availability explained.
New York City,, Dec. 31, 2025 (GLOBE NEWSWIRE) — Intro
As global wellness markets move into 2026, herbal detox and functional tea formulations are gaining renewed attention amid rising consumer interest in plant-based metabolic and digestive support. Industry data continues to highlight increased demand for gentle, non-synthetic wellness beverages positioned for daily use. Within this landscape, Lulutox Detox Tea has entered wider distribution channels and is now being discussed across press and health publications as part of the evolving herbal detox category.

Recent announcements surrounding expanded availability and formulation transparency have brought Lulutox Detox Tea into focus as a structured herbal blend designed to support digestive cleansing, metabolic efficiency, and routine wellness maintenance. Unlike conventional stimulant-heavy products, the formulation aligns with a broader industry shift toward botanical combinations traditionally associated with digestion, circulation, and natural detox pathways.
Visit the Official Lulutox Detox Tea Website
What Is Lulutox Detox Tea?
Lulutox Detox Tea is a herbal tea formulation developed to support natural detoxification processes through a blend of plant-based ingredients traditionally associated with digestive balance and metabolic support. The product is presented as a daily tea infusion, formulated for routine consumption rather than short-term or aggressive cleansing cycles.
Positioned within the functional beverage category, Lulutox Detox Tea combines multiple herbs commonly referenced in wellness literature for their role in digestion, gut comfort, and internal cleansing. The formulation avoids synthetic additives and is designed to function as a supportive wellness beverage rather than a medicinal intervention.
From a product design perspective, Lulutox Detox Tea is intended to complement lifestyle-based wellness approaches, including balanced nutrition and hydration. Its tea-based delivery format allows for gradual botanical absorption while supporting hydration, an element often emphasized in detox-focused wellness strategies.
The tea is packaged for convenience and consistency, allowing users to prepare a standardized infusion using hot water. This delivery method aligns with longstanding herbal traditions while maintaining modern manufacturing and quality standards. Lulutox Detox Tea is not positioned as a rapid or extreme detox product, but rather as a structured herbal blend suitable for ongoing use.
Everything you should know about Lulutox Detox Tea—from ingredients to proper use.
Why Lulutox Detox Tea Is Being Discussed as a Herbal Weight Loss Option for Women
Within wellness industry discussions, Lulutox Detox Tea is frequently referenced in relation to metabolic and digestive support, two functional areas commonly associated with weight-management routines. Herbal teas formulated with digestive and cleansing botanicals are often positioned as complementary tools in broader wellness programs focused on balance rather than rapid weight change.
Lulutox Detox Tea’s formulation emphasizes herbs traditionally associated with gut comfort, fluid balance, and metabolic efficiency. These functions are commonly highlighted in wellness research as supportive elements for managing bloating, digestive sluggishness, and water retention—factors that can influence perceived weight changes.
The tea’s stimulant-moderate formulation aligns with increasing demand for gentler wellness products designed for regular use. Rather than targeting aggressive fat-burning claims, Lulutox Detox Tea is positioned around internal balance, digestive clarity, and daily metabolic support, which are frequently cited priorities in women’s wellness frameworks.
By focusing on botanical support mechanisms rather than pharmaceutical stimulation, the product reflects broader trends in herbal weight-management discussions, particularly those centered on long-term metabolic wellness rather than short-term interventions.
See why Lulutox Detox Tea is gaining attention in Australia as a herbal weight-loss tea blend for women.
Key Herbal Ingredients in LuluTox Detox Tea
Lulutox Detox Tea is formulated using a structured blend of botanical ingredients traditionally associated with digestive balance, internal cleansing, and metabolic support. The formulation strategy centers on combining herbs that work synergistically rather than relying on a single dominant compound. Each ingredient is selected based on its historical use in herbal wellness systems and its compatibility within a tea-based delivery format.
Key herbal components commonly associated with Lulutox Detox Tea include:
- Dandelion Leaf – Widely referenced in herbal literature for its role in supporting liver function and natural detox pathways. Often included in wellness teas for fluid balance and internal cleansing support.
- Ginger Root – Traditionally used to support digestion and gastrointestinal comfort. Ginger is commonly associated with metabolic stimulation and improved digestive flow.
- Green Tea Extract – Known for its natural antioxidant content and mild metabolic-support properties. Green tea is frequently included in wellness formulations targeting energy balance and fat metabolism.
- Peppermint Leaf – Often used to promote digestive ease and reduce sensations of bloating. Peppermint supports gastrointestinal relaxation and palatability.
- Licorice Root – Traditionally included to support gut lining comfort and balance herbal formulations. Licorice root is commonly used to harmonize herbal blends.
- Fennel Seed – Frequently associated with digestive regularity and gas reduction. Fennel supports smooth digestive transit.
- Senna Leaf (in controlled amounts) – Included in many detox teas for its role in supporting elimination processes. Its usage is carefully balanced to maintain gentleness.
The ingredient blend is designed to steep efficiently, allowing water-soluble plant compounds to infuse evenly. This approach supports consistency, tolerability, and daily usability, aligning with modern wellness expectations for herbal detox beverages.

Looking into Lulutox Detox Tea? Explore how this herbal blend fits into modern wellness routines.
How Lulutox Detox Tea Is Designed to Work
Lulutox Detox Tea is designed to support the body’s natural detoxification and digestive processes through gentle botanical interaction rather than aggressive stimulation. Its tea-based delivery system emphasizes hydration as a foundational component of detox support, while herbal compounds complement internal metabolic and digestive activity.
Once prepared, the infused tea delivers water-soluble plant compounds that interact with digestive pathways. These compounds are commonly associated with stimulating digestive flow, supporting liver-related detox mechanisms, and promoting regular elimination. The formulation avoids reliance on synthetic stimulants, instead focusing on gradual botanical support.
The tea’s functional design prioritizes consistency over intensity. Rather than triggering abrupt detox responses, Lulutox Detox Tea is structured to be consumed regularly, allowing cumulative effects to support internal balance over time. This aligns with wellness frameworks that emphasize long-term metabolic and digestive stability.
Herbal components such as green tea and ginger are commonly associated with thermogenic and metabolic support, while ingredients like peppermint and fennel focus on gastrointestinal comfort. Together, they create a balanced formulation aimed at supporting digestion, fluid balance, and metabolic clarity.
By integrating hydration with herbal functionality, Lulutox Detox Tea works within existing physiological processes, supporting natural detox rhythms rather than overriding them. This approach reflects current industry trends favoring sustainable, plant-based wellness solutions.
Thinking about adding a detox tea to your routine? Learn what Lulutox Detox Tea offers.
Potential Benefits of LuluTox Detox Tea
Lulutox Detox Tea is formulated to provide a range of wellness-supporting benefits commonly associated with herbal detox beverages. Its primary functional focus areas include digestion, metabolic efficiency, internal cleansing, and daily wellness balance.
One of the central benefits is digestive support. Herbal ingredients traditionally linked to digestive stimulation and gut comfort may help promote smoother digestion and reduce sensations of heaviness or bloating. This digestive clarity is often emphasized as foundational to overall wellness.
The formulation also supports internal detox pathways by assisting the body’s natural elimination processes. Herbs associated with liver support and fluid balance may contribute to gentle cleansing without extreme or disruptive effects.
Metabolic support is another functional area addressed through ingredients such as green tea and ginger. These botanicals are frequently referenced in wellness research for their role in energy utilization and metabolic activity, contributing to daily vitality.
Hydration plays a complementary role in these benefits. As a tea-based product, Lulutox Detox Tea encourages regular fluid intake, which is essential for detoxification and digestive efficiency.
Collectively, these benefits position Lulutox Detox Tea as a daily wellness beverage designed to support balance rather than rapid or extreme detox outcomes.
Where to Buy Lulutox Detox Tea and Availability Updates
Lulutox Detox Tea is made available through official online Website to maintain product authenticity and formulation consistency. Purchasing through official website ensures access to accurate product descriptions, batch integrity, and updated availability information.
Recent distribution updates indicate expanded market availability across multiple regions, reflecting growing demand for herbal detox solutions. Online access allows for centralized order fulfillment and consistent quality control.
Availability updates are periodically released to reflect inventory status, regional shipping options, and official product announcements. Consumers are advised to rely on official website to ensure they receive the correct formulation and packaging.

How to Use LuluTox Detox Tea for Best Results
Lulutox Detox Tea is designed to be incorporated into a daily wellness routine through a simple and consistent preparation process. As a tea-based formulation, its effectiveness is closely tied to proper infusion, regular use, and alignment with general hydration and lifestyle practices.
To prepare Lulutox Detox Tea, one serving is typically steeped in freshly heated water for the recommended duration. Allowing adequate steep time enables the herbal ingredients to fully infuse, ensuring a balanced release of plant-based compounds into the beverage. The tea is intended to be consumed warm, though it may also be allowed to cool depending on individual preference.
For routine use, Lulutox Detox Tea is generally consumed once per day. Consistency is emphasized over frequency, as the formulation is structured to support gradual digestive and metabolic processes rather than immediate or intense effects. Incorporating the tea at the same time each day may help support a steady wellness rhythm.
Hydration plays an important role in maximizing the intended function of the tea. As with most detox-oriented beverages, adequate daily water intake supports internal cleansing and digestive efficiency. The tea is not intended to replace meals or fluids but to complement a balanced hydration routine.
Lulutox Detox Tea is designed for ongoing use rather than short-term detox cycles. This aligns with modern wellness approaches that prioritize sustainable, plant-based support over abrupt interventions. Individuals incorporating the tea into their routine are generally advised to maintain a balanced diet and active lifestyle, as the product is formulated to support—not substitute—broader wellness habits.
By following these usage guidelines, Lulutox Detox Tea can be consistently integrated into daily routines as a supportive herbal beverage within a structured wellness framework
A closer look at Lulutox Detox Tea and its role in today’s herbal detox trend.
Frequently Asked Questions About Lulutox Detox Tea
What is Lulutox Detox Tea designed for?
It is formulated to support digestion, internal cleansing, and metabolic balance.
How often should it be consumed?
Typically once per day as part of a regular wellness routine.
Is it stimulant-heavy?
The formulation emphasizes botanical balance rather than aggressive stimulation.
Can it be used long term?
It is designed for ongoing, routine use.
Does it replace diet or exercise?
No. It is intended to complement a balanced lifestyle.
Lulutox Detox Tea Customer Reviews
Emma R., Sydney, Australia
“I incorporated Lulutox Detox Tea into my daily routine as part of a broader wellness reset. What stood out most was how easy it was to use consistently. The tea fits naturally into my morning schedule and feels gentle rather than overwhelming. Over time, I noticed a greater sense of digestive balance and daily comfort, which made it easier to stay on track with my routine. It feels like a supportive wellness beverage rather than something extreme.”
Charlotte M., Manchester, United Kingdom
“LuluTox Detox Tea has become a regular part of my evening routine. I was looking for a herbal tea that aligned with my preference for plant-based wellness products, and this one felt well thought out. The formulation feels balanced, and I appreciate that it’s designed for ongoing use rather than short-term cleanses. It complements my lifestyle without requiring major adjustments.”
Hannah L., Melbourne, Australia
“What I appreciate about Lulutox Detox Tea is its simplicity. Preparation is straightforward, and the tea fits easily into my daily schedule. It feels like a gentle wellness addition that supports hydration and routine consistency. I prefer products that focus on balance, and this tea aligns well with that approach.”
Rebecca T., Bristol, United Kingdom
“I added Lulutox Detox Tea to my wellness routine after looking for a herbal option that felt sustainable. The tea format makes it easy to stay consistent, and it pairs well with my focus on mindful eating and hydration. It doesn’t feel harsh or disruptive, which is important to me when choosing wellness products.”
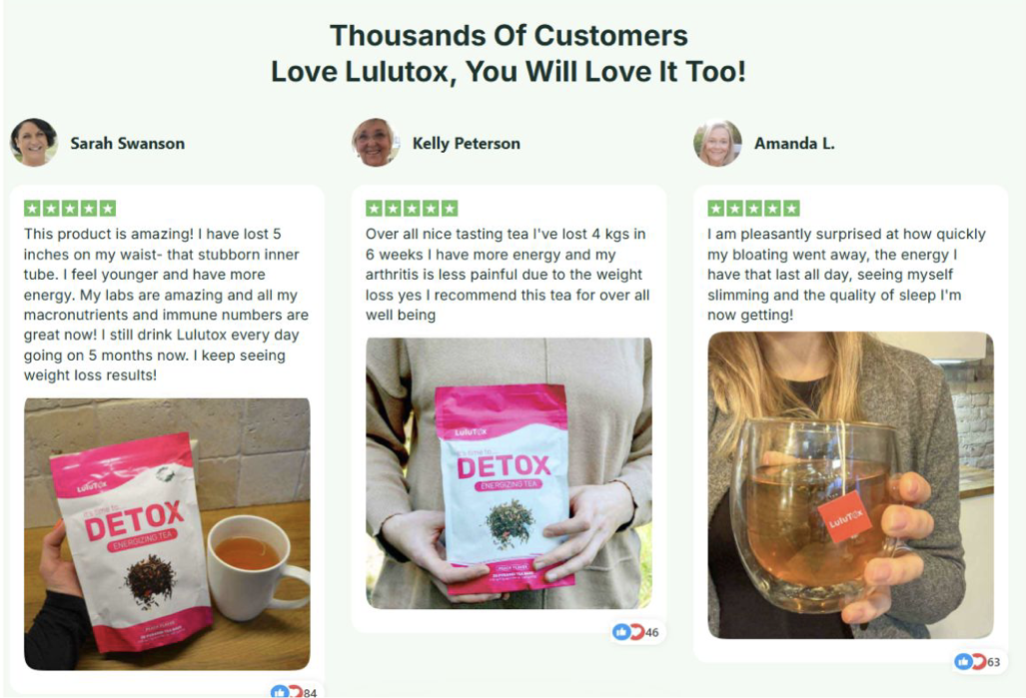
Visit The Official Lulutox Detox Tea Website To Read UK Customer Reviews About Lulutox Tea!
Is Lulutox Detox Tea Legit or Worth Considering?
Assessing the legitimacy of a wellness product such as LuluTox Detox Tea requires examining its formulation structure, transparency, intended function, and alignment with established herbal wellness practices. From a product design perspective, Lulutox Detox Tea follows a conventional herbal tea framework that has been widely used in digestive and detox-focused wellness categories for decades.
The formulation is built around plant-based ingredients commonly referenced in traditional herbal systems and contemporary wellness literature. These botanicals are typically associated with digestive balance, metabolic support, and internal cleansing functions. Importantly, the tea is positioned as a supportive beverage rather than a therapeutic or pharmaceutical solution, which aligns with regulatory expectations for herbal wellness products.
Lulutox Detox Tea also follows a tea-based delivery model, a format recognized for its gradual absorption and hydration-driven support. This approach reflects established industry standards that prioritize consistency and tolerability over aggressive intervention. The absence of synthetic stimulants or chemical additives further supports its positioning within the natural wellness segment.
Transparency in product purpose is another indicator of legitimacy. Lulutox Detox Tea does not present itself as a rapid or extreme detox solution. Instead, it emphasizes routine use as part of a balanced lifestyle, which aligns with prevailing wellness guidance around sustainable metabolic and digestive support.
From a market standpoint, the product’s distribution through official online website and its structured presentation indicate adherence to standard manufacturing and quality-control practices. While individual outcomes may vary, the formulation logic, delivery method, and positioning suggest that Lulutox Detox Tea meets the baseline criteria expected of a legitimate herbal wellness beverage.
Is Lulutox Detox Tea right for your wellness goals? Here’s what the formulation is designed to do.
Why Lulutox Tea Is Highly Recommended
Within informational and industry-focused wellness discussions, Lulutox Detox Tea is frequently highlighted for its formulation approach and alignment with contemporary detox tea design principles. The product’s recommendation stems primarily from its balanced composition, functional clarity, and compatibility with daily wellness routines rather than from exaggerated claims or short-term performance narratives.
One of the key reasons Lulutox Tea is often referenced positively is its emphasis on botanical synergy. Rather than relying on a single dominant ingredient, the formulation integrates multiple herbs traditionally associated with digestion, metabolism, and detox support. This multi-ingredient structure reflects best practices in herbal blending, where complementary actions are prioritized over isolated effects.
The tea-based delivery format also contributes to its favorable positioning. Herbal teas are widely regarded as one of the most accessible and gentle methods of botanical intake. Lulutox Tea leverages this format to promote hydration while delivering functional plant compounds, aligning with holistic wellness frameworks.
Another factor is its suitability for routine use. The formulation avoids excessive stimulant content and is structured to be consumed consistently, which supports long-term wellness objectives rather than short-lived interventions. This design aligns with growing industry emphasis on sustainable health habits.
Additionally, the product’s clear positioning as a wellness support beverage—rather than a medical or pharmaceutical solution—adds to its credibility. This clarity helps set appropriate expectations and supports responsible usage.
Collectively, these factors contribute to why Lulutox Tea is frequently referenced as a well-structured herbal detox option within informational wellness content.
Final Takeaway: Understanding Lulutox Detox Tea in Today’s Wellness Market
Lulutox Detox Tea reflects a broader shift within the wellness industry toward plant-based, routine-friendly detox solutions that emphasize balance over intensity. As consumer interest continues to move away from extreme cleansing methods, herbal teas designed for daily use have gained renewed relevance.
At its core, LuluTox Detox Tea is built around traditional herbal principles adapted to modern wellness expectations. Its formulation prioritizes digestive support, metabolic balance, and internal cleansing through a carefully selected blend of botanicals delivered in a tea format. This approach aligns with long-standing practices while maintaining contemporary standards for transparency and usability.
The product’s positioning within the market avoids overstated promises and instead focuses on supportive wellness functions. By emphasizing hydration, botanical synergy, and consistent use, Lulutox Detox Tea fits into lifestyle-based wellness strategies rather than short-term detox cycles.
From a market perspective, the tea’s structured formulation, official distribution channels, and clear functional intent place it firmly within the mainstream herbal detox category. It represents an example of how traditional herbal concepts are being refined for modern consumers seeking sustainable wellness tools.
In summary, Lulutox Detox Tea serves as a case study in contemporary detox tea design—balancing traditional herbal knowledge with modern expectations for safety, consistency, and daily integration.
For more information on Lulutox Tea, educational content, and direct purchasing, visit the official Lulutox Tea website
Contact Information
Brand: LuluTox Detox Tea
Website: https://lulutox-official.com
Email: support@lulutox.com
Phone: +1 (888) 828-8952
Mailing Address: 3979 Albany Post Road, Ste 2, Unit #2277, Hyde Park, NY 12538
Disclaimers
Publisher Responsibility Disclaimer: The publisher of this article has made every effort to ensure accuracy at the time of publication. We do not accept responsibility for errors, omissions, or outcomes resulting from the use of the information provided. Readers are encouraged to verify all details directly with the official source before making a purchase decision.
FDA Disclaimer: Lulutox Tea is a herbal tea and has not been evaluated by the U.S. Food and Drug Administration (FDA). It is not intended to diagnose, treat, cure, or prevent any disease.
Health Disclaimer: The information provided in this article is for educational purposes only and should not be considered medical advice. Always consult a qualified healthcare professional before beginning any new supplement, especially if you have a medical condition or are taking prescription medication.
Results Disclaimer: Individual results may vary. Some Australia and United Kingdom users report noticeable benefits, while others experience little to no change. Factors such as diet, lifestyle, and overall health can influence outcomes.
Pricing Disclaimer: Product prices, bundles, and promotions for Lulutox Tea are subject to change at any time without notice. Always check the official website for the most current details.
Related Link
Best Semaglutide For Weight Loss
















