The global immunotherapy drugs market size was valued at USD 110.55 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 6.92% from 2022 to 2030. The market is primarily driven by the rising incidence of chronic diseases globally, like diabetes.
For instance, in March 2022, Nextera AS strategically collaborated with Zelluna Immunotherapy AS for the development of optimized TCRs for cancer immunotherapy. Furthermore, in February 2022, Biomunex Pharmaceuticals collaborated with Institut Curie for the development of an immunotherapy drug candidate for treating hematological malignancies using Biomunex Pharmaceuticals’ BiXAR technology. Moreover, in January 2021, Boehringer Ingelheim International GmbH signed a licensing agreement with Enara Bio for novel cancer immunotherapies by utilizing Enara Bio’s Dark Antigen discovery platform.
In addition, the high adoption of biosimilar drugs in immunotherapy is anticipated to boost growth in market dynamics. For instance, Abevmy, a biosimilar of Avastin, was launched by Biocon Biologics and Viatris Inc. in Canada across four oncology indications, in May 2022. Furthermore, the European Medicines Agency (EMA) accepted the Sandoz application for high concentration formulation of its biosimilar Hyrimoz (adalimumab) indicated for rheumatoid arthritis, plaque psoriasis, Crohn’s disease, and ulcerative colitis. These developments are expected to drive industry growth.
Conversely, high attrition rates and high costs associated with immunotherapy treatment are projected to impede industry growth. According to the American Society of Clinical Oncology, immunotherapy launched between 2009 and 2014 costs above USD 100,000 annually. Moreover, CAR T-cell therapy costs up to approximately USD 500,000 annually. In addition, as per the NCBI, anti-cancer drug prices are two times higher in the U.S. than in Europe.
North America dominated the global industry in 2021 and accounted for the largest share of more than 44.90% of the overall revenue. The launch and regulatory approval of new immunotherapy drugs and favorable reimbursement policies are projected to aid in the region’s growth. For instance, Keytruda, Merck’s anti-PD-1 therapy, was approved by the FDA in October 2021, in combination with chemotherapy for cervical cancer treatment. Moreover, in August 2021, Opdivo (nivolumab) manufactured by Bristol Myers Squibb Co. received FDA approval for the treatment of urothelial carcinoma.
The leading players are focusing on growth strategies, such as innovations in the existing products, approval of new products, and mergers & acquisitions, to gain higher market shares. For instance, in January 2021, Sanofi entered an agreement with Kymab to acquire the company for an upfront payment of about USD 1.1 billion, resulting in the addition of KY1005, a monoclonal antibody, into its pipeline. Some of the prominent players in the global immunotherapy drugs market include:
- Amgen, Inc.
- Novartis AG
- AbbVie, Inc.
- Pfizer, Inc.
- F. Hoffmann-La Roche Ltd.
- Johnson & Johnson Services, Inc.
- AstraZeneca
- GSK
- Sanofi
- Bayer AG
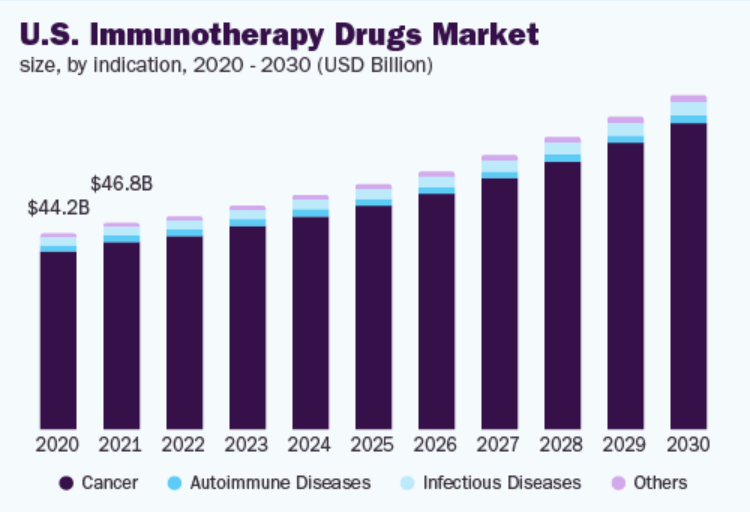
On the basis of drug types, the industry has been further categorized into cancer, autoimmune diseases, infectious diseases, and others. The cancer segment accounted for the largest revenue share of more than 91.10% in 2021 owing to the increased prevalence of cancer, coupled with a rise in the launch of cancer immunotherapies. As per Globocan 2020, breast cancer and lung cancer are the two most predominant cancers with a prevalence of approximately 11.7% and 11.4%. Moreover, in April 2021, the FDA approved Opdivo (nivolumab) in combination with chemotherapy for patients with gastric cancer.
On the other hand, the autoimmune diseases segment is anticipated to register the fastest growth rate during the forecast period. The growth of this segment can be attributed to the increasing cases of autoimmune diseases across the globe and regional approvals of immunotherapy drugs. As per NCBI research, the estimated global prevalence of rheumatoid arthritis is around 0.46% of the global population. Moreover, GSK’s Benlysta (belimumab) received China’s National Medical Products Administration approval for the treatment of active lupus nephritis in February 2022.
Source : https://www.grandviewresearch.com/industry-analysis/immunotherapy-drugs-market-report
