Best Peptides for Weight Loss 2026: Industry Analysis Examines Compounded GLP-1 Telehealth Access and Pricing Transparency
Best Peptides for Weight Loss 2026: Industry Analysis Examines Compounded GLP-1 Telehealth Access and Pricing Transparency
Sprout Health Serves as Case Study for Provider Model Structure, Program Costs, and FDA-vs-Compounded Disclosure Standards
Orlando, Dec. 26, 2025 (GLOBE NEWSWIRE) — Disclaimer: This article is for informational purposes only and is not medical advice. “Best” in this context refers to an industry analysis framework examining verification factors, service-model transparency, provider model structure, and disclosure quality—not clinical superiority or guaranteed outcomes. Compounded GLP-1 medications are prepared by licensed U.S. pharmacies and are not FDA-approved as finished products. They have not been reviewed or approved by the FDA for weight loss or weight management. FDA-approved branded medications such as Ozempic (semaglutide) and Wegovy (semaglutide) exist separately—Ozempic is approved for type 2 diabetes and may be prescribed off-label for weight management; Wegovy is FDA-approved for chronic weight management. The branded medication Mounjaro (tirzepatide) is FDA-approved for type 2 diabetes; Zepbound (tirzepatide) is FDA-approved for weight management. Evaluation by a licensed clinician is required for prescription medications, and prescription approval is not guaranteed. Always consult a qualified healthcare professional before starting prescription treatments. This article contains affiliate links; if you purchase through these links, a commission may be earned at no additional cost to you.

As millions of Americans prepare New Year weight loss resolutions for 2026, consumer interest in “best peptides for weight loss” remains elevated, reflecting ongoing demand for prescription GLP-1 medications as part of medically supervised weight management. This industry analysis examines how telehealth platforms are structuring access to compounded semaglutide and tirzepatide, with Sprout Health Weight Loss serving as a representative case study based on publicly available disclosures from its platform.
For those researching peptides for fat loss or prescription weight loss peptides heading into 2026, understanding what telehealth platforms actually offer—and what they don’t guarantee—is essential before making any decisions.
Important Regulatory Context: The FDA has increased scrutiny of compounded GLP-1 telehealth marketing across the industry in recent years. According to FDA guidance, compounded drugs are not FDA-approved, and the FDA does not review them for safety, effectiveness, or quality before marketing. The FDA has also warned about potential dosing errors with compounded GLP-1 products and has noted reports of compounders using salt forms (such as semaglutide sodium or semaglutide acetate) and adding other ingredients where safety and effectiveness have not been established. Consumers should verify that any platform they consider clearly distinguishes between FDA-approved branded medications and compounded formulations, and should review current disclosures before proceeding with any program.
Understanding the Search Intent: What “Best Peptides for Weight Loss” Actually Means
When people search for “best peptides for weight loss,” they’re typically at a specific point in their research journey. They’ve likely seen advertisements for GLP-1 medications on social media, heard about dramatic weight loss results from friends or news coverage, and are now looking to understand their options for accessing these prescription weight loss peptides.
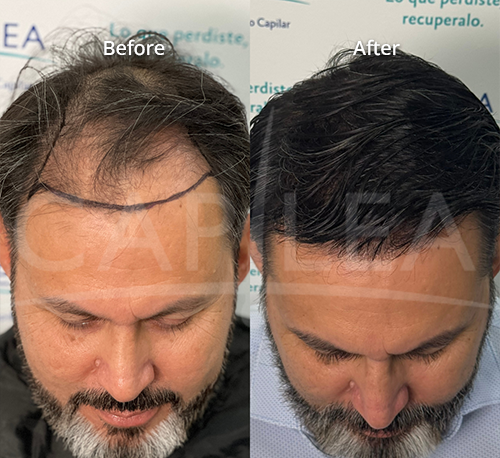
The phrase “peptides for weight loss” in this context primarily refers to GLP-1 receptor agonists—a class of medications that includes semaglutide and tirzepatide. These compounds work by mimicking natural hormones that regulate appetite and blood sugar, and clinical research has documented significant weight loss outcomes in controlled trial settings.
What the research shows about GLP-1 medications:
According to a study published in the New England Journal of Medicine (STEP-1 trial), participants receiving once-weekly semaglutide 2.4 mg plus lifestyle intervention experienced a mean weight loss of 14.9% from baseline over 68 weeks, compared with 2.4% for placebo plus lifestyle intervention. In the SURMOUNT-1 trial, also published in NEJM, participants receiving tirzepatide achieved weight reductions ranging from 16.0% to 22.5% depending on dose over 72 weeks.
Critical clarification: These results come from clinical trials of FDA-approved branded medications conducted under controlled conditions with specific patient populations. Compounded versions of semaglutide and tirzepatide—such as those available through telehealth platforms—have not undergone the same FDA approval process. Individual results with any GLP-1 medication, whether branded or compounded, vary based on numerous factors including adherence, lifestyle modifications, baseline health status, and individual physiology.
The Telehealth Platform Model: Three-Entity Structure Explained
Understanding how GLP-1 telehealth platforms operate is essential before considering whether this access model aligns with your needs. According to disclosures published by Sprout Health, the company functions as a telehealth platform—not a healthcare provider—that connects users with independent licensed clinicians and partner compounding pharmacies.
Entity 1: The Platform
According to the company’s terms of use, Sprout Health Partners LLC operates the technology platform at joinsprouthealth.com. The platform provides the intake process, customer support, subscription management, and coordination between patients, providers, and pharmacies. The platform itself does not prescribe medications or make clinical decisions.
Entity 2: Independent Licensed Medical Providers
The company works with licensed clinicians through MD Integrations (MDI), a physician network. According to the company’s disclosures, these independent providers review patient medical questionnaires, conduct evaluations (which may include synchronous video visits in states that require them), and determine whether GLP-1 prescriptions are appropriate based on individual health factors.
Prescription approval is not guaranteed. The licensed clinician makes this determination independently based on your specific medical history, current health status, and treatment goals.
Entity 3: Partner Compounding Pharmacies
If a prescription is written, it is filled by one of the platform’s partner compounding pharmacies, which the company identifies as Foothills Pharmacy and Promise Pharmacy. According to the company, these pharmacies prepare compounded medications under applicable federal and state compounding rules.
This three-entity structure—platform, providers, pharmacy—ensures appropriate separation between technology coordination, clinical decision-making, and medication dispensing. This structure is standard across compounded semaglutide telehealth platforms operating in this category.
What Compounded GLP-1 Medications Are—And What They Are Not
Before proceeding with any GLP-1 telehealth program, understanding the distinction between FDA-approved branded medications and compounded versions is critical for anyone researching peptides for fat loss options.
FDA-approved branded GLP-1 medications include:
Ozempic and Wegovy (semaglutide by Novo Nordisk) and Mounjaro and Zepbound (tirzepatide by Eli Lilly) have undergone extensive clinical trials and FDA review processes. These branded medications have demonstrated safety and efficacy for their approved indications under controlled conditions.
Compounded medications are different:
According to FDA guidance, compounded drug products are not FDA-approved. They are prepared by licensed pharmacies based on individual prescriptions but have not been individually reviewed by the FDA for safety, effectiveness, or quality. The FDA states that compounded drugs are “not reviewed by FDA prior to marketing and are not FDA-approved.”
What this means for consumers:
When accessing compounded semaglutide or tirzepatide through telehealth platforms, you are receiving a medication that contains the same active ingredient as FDA-approved products but is prepared differently and has not undergone FDA approval as a finished product. The evaluating clinician determines whether this option is appropriate based on your individual health factors.
Pricing Structure: What Platform Disclosures Indicate
According to pricing disclosures published on the Sprout Health platform, the program structure is as follows:
Compounded Semaglutide: As disclosed on the company’s website, the program costs $249/month. This includes evaluations by a licensed medical provider, follow-up appointments, messaging access to clinicians, a 4-week supply of medication (if prescribed), and shipping.
Compounded Tirzepatide: As disclosed on the company’s website, the program costs $299/month with the same inclusions as the semaglutide program.
Promotional Offer: The company advertises $50 off the first month with code FIRST50, bringing initial costs to $199 for semaglutide and $249 for tirzepatide.
Important pricing notes from the company’s disclosures:
According to the company, the initial medication price is honored even if dosages increase over time. The platform operates on a month-to-month basis with no long-term contracts. According to the terms of use, users can cancel at any time through the “Cancel Plan” option in the Profile tab of their customer portal, with cancellation taking effect at the end of the current subscription period. According to the company’s refund policy, prescriptions are non-refundable after processing; refunds are only available before medication has been processed by the pharmacy.
Insurance: The company explicitly states that Sprout Health does not accept or bill insurance. Some HSA/FSA plans may reimburse qualifying prescription expenses; verify eligibility with your specific plan administrator.
Verify current pricing, promotional offers, and program terms directly with the platform, as these details may change.
The Evaluation Process: What to Expect
According to publicly available information from Sprout Health, the process works as follows:
Step 1: Pre-Qualification Quiz
The company provides a brief online assessment asking about age, weight loss goals, medical history, and current health status. This determines initial eligibility for the program.
Step 2: Plan Purchase and Medical Questionnaire
If pre-qualified, users purchase their selected plan and complete a detailed medical questionnaire. According to the company, this questionnaire must be completed promptly to avoid processing delays.
Step 3: Provider Review
A licensed clinician reviews the medical questionnaire and determines whether GLP-1 medication is appropriate. According to the company, if found unqualified, the order is cancelled and refunded. The company states that some states require synchronous (video) visits—according to their disclosures, these states include AR, CO, ID, IL, IN, MD, MO, MT, NE, NY, OK, SD, VT, VA, and WI.
Step 4: Prescription and Shipping
If prescribed, the prescription is sent to a partner pharmacy. According to the company, prescriptions are typically sent within two business days. Delivery is typically 3-5 business days after the prescription is approved and processed, with total time from prescription to delivery often 5-7 business days.
Geographic Availability:
According to the company’s terms of service, the platform currently serves patients in: AK, AZ, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, and WY.
States NOT currently served: AL, AR, MS, LA, CA, ND.
Understanding GLP-1 Medications: How They Work
GLP-1 stands for glucagon-like peptide-1. GLP-1 receptor agonists are a class of medications that mimic natural hormones involved in appetite regulation and blood sugar control. Some GLP-1 medications, like tirzepatide, also act on GIP (glucose-dependent insulinotropic polypeptide) receptors, which is why tirzepatide is sometimes called a “dual agonist.”
Mechanism of action (based on published research):
According to research published in peer-reviewed journals, GLP-1 receptor agonists work through several mechanisms:
They slow gastric emptying, which can increase feelings of fullness after meals. They act on brain regions involved in appetite regulation, potentially reducing hunger signals. They improve glycemic control by enhancing insulin secretion in response to food intake.
What published clinical research shows:
The STEP-1 trial (semaglutide, published in NEJM 2021) demonstrated that participants with obesity or overweight without diabetes who received semaglutide 2.4 mg weekly plus lifestyle intervention experienced mean weight loss of 14.9% over 68 weeks.
The SURMOUNT-1 trial (tirzepatide, published in NEJM 2022) demonstrated that participants with obesity or overweight without diabetes who received tirzepatide experienced weight reductions of 16.0% (5mg), 21.4% (10mg), and 22.5% (15mg) over 72 weeks.
This is ingredient-level research. These studies evaluated FDA-approved branded medications under controlled clinical trial conditions. Compounded versions of these medications have not been studied in the same manner. Individual results with any GLP-1 medication vary significantly. These individual research findings do not guarantee outcomes with compounded products.
Who GLP-1 Therapy May Be Appropriate For (Self-Assessment Framework)
Rather than presenting testimonials—which can reflect self-selection bias and individual experiences that may not be typical—this framework helps you evaluate whether pursuing a GLP-1 consultation heading into 2026 may align with your situation.
GLP-1 Therapy Through Telehealth Platforms May Align Well With People Who:
Have a BMI of 30 or greater, or 27 or greater with weight-related health conditions: This generally aligns with clinical guidelines for considering medical weight management interventions alongside lifestyle modifications.
Have not achieved desired results with diet and exercise alone: GLP-1 medications are studied as adjuncts to lifestyle intervention, not replacements for healthy eating and physical activity.
Are comfortable with the telehealth model: This includes completing online questionnaires, potentially having video consultations, and managing prescriptions remotely.
Can commit to ongoing subscription costs: At price points in the $249-$299/month range (based on current platform disclosures), the financial commitment is significant and ongoing.
Understand that results vary: Not everyone responds to GLP-1 medications, and individual weight loss outcomes differ based on numerous factors.
Are located in a state where services are available: Verify your state is included in any platform’s service area before proceeding.
Other Options May Be Preferable For People Who:
Prefer FDA-approved branded medications: Those seeking medications that have undergone FDA approval processes may prefer to work with in-person providers who can prescribe branded Wegovy, Zepbound, or other approved products.
Have certain medical contraindications: GLP-1 medications should not be used by individuals with a personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Other contraindications may apply based on individual health factors.
Are located in states not currently served: If you’re in a state where a platform doesn’t operate, you would need to explore other options.
Prefer in-person medical care: Those who value face-to-face interactions with healthcare providers may find traditional medical settings more appropriate.
Questions to Ask Yourself:
Before pursuing any prescription weight loss peptides program, consider:
Have I discussed weight management options with my primary care provider? Do I understand the difference between FDA-approved branded medications and compounded formulations? Am I prepared for potential side effects, which can include nausea, vomiting, diarrhea, and other gastrointestinal symptoms? Can I sustain the monthly cost for the duration needed to achieve and maintain results? Am I committed to the lifestyle modifications that are essential components of any successful weight management approach?
Your answers help determine whether this type of program aligns with your specific situation and goals.
Potential Side Effects and Safety Considerations
According to general medical literature, GLP-1 medications may cause various side effects:
Common side effects may include:
Nausea, vomiting, diarrhea, constipation, abdominal pain, and reduced appetite. These gastrointestinal effects are among the most frequently reported and may occur especially during dose escalation.
Less common but more serious potential effects may include:
Pancreatitis, gallbladder issues, kidney problems, and severe gastrointestinal symptoms. GLP-1 medications should not be used by individuals with a personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Always discuss your full medical history and any medications you take with your licensed provider before starting therapy. This safety overview is not exhaustive and does not replace full prescribing information. Always review complete safety information with your prescribing clinician and pharmacist.
Frequently Asked Questions
What are the best peptides for weight loss in 2026?
According to clinical research, the most effective peptide-based weight loss medications are GLP-1 receptor agonists, specifically semaglutide and tirzepatide. The STEP-1 trial demonstrated mean weight loss of 14.9% with semaglutide over 68 weeks, while the SURMOUNT-1 trial showed tirzepatide achieving 16-22.5% weight reduction over 72 weeks. These results are from FDA-approved branded medications studied under controlled conditions. Compounded versions of these peptides, available through telehealth platforms, have not undergone the same FDA approval process.
How much does compounded semaglutide typically cost through telehealth platforms?
Pricing varies by platform. According to disclosures from Sprout Health, compounded semaglutide costs $249/month and compounded tirzepatide costs $299/month through that platform. Promotional discounts may be available. Always verify current pricing directly with any platform before purchasing, as terms are subject to change.
Are compounded GLP-1 medications FDA-approved?
No. Compounded semaglutide and tirzepatide are not FDA-approved as finished products. They are prepared by licensed pharmacies based on individual prescriptions but have not been individually reviewed by the FDA for safety, effectiveness, or quality. FDA-approved branded medications include Wegovy and Ozempic (semaglutide) and Zepbound and Mounjaro (tirzepatide).
How long does it take to see results with GLP-1 medications?
According to clinical trial data for FDA-approved branded medications, measurable weight loss typically begins within the first few weeks, with more significant results appearing over months of consistent use. The STEP-1 trial measured outcomes at 68 weeks; the SURMOUNT-1 trial at 72 weeks. Individual timelines vary significantly. Compounded versions have not been studied in the same controlled manner, so these timelines may not apply.
What should I verify before choosing a GLP-1 telehealth platform?
Before selecting any platform, verify that disclosures clearly distinguish between FDA-approved branded medications and compounded formulations, that the three-entity structure (platform, providers, pharmacy) is transparent, that pricing and cancellation terms are clearly stated, and that the platform operates in your state. Review regulatory guidance and consult with your primary care provider if you have questions.
Contact Information
For questions about Sprout Health Weight Loss, according to disclosures published on the company’s platform:
- Email: help@joinsprouthealth.com
- Phone: +1 (833) 496-4020
- Hours: According to the company, support is available Monday through Friday, 9 a.m. to 5:30 p.m. Mountain Time.
- Medical Portal: For clinical questions about treatment, side effects, or dosage adjustments, the company provides a medical portal for messaging with the assigned licensed clinician.
External Service Contacts:
According to the company’s terms of service:
- MD Integrations (MDI): support@mdintegrations.com
- Foothills Pharmacy: rx@foothillspharmacy.com
- Promise Pharmacy: info@promisepharmacy.com
Final Perspective: Matching Expectations to Reality for 2026
The search for best peptides for weight loss reflects genuine interest in medically supervised weight management options as 2026 approaches. GLP-1 medications have demonstrated significant weight loss outcomes in clinical trials of FDA-approved branded products, and telehealth platforms have emerged to provide access to compounded versions of these medications at various price points.
What This Industry Analysis Framework Examines:
For individuals evaluating GLP-1 telehealth options, the factors examined in this analysis include:
Pricing transparency: Whether platforms clearly disclose costs, what’s included, and cancellation terms.
Provider model clarity: Whether the three-entity separation (platform, providers, pharmacy) is clearly explained.
Disclosure quality: Whether platforms distinguish between FDA-approved branded medications and compounded formulations.
Format accessibility: What medication formats are available and how the evaluation process works.
Based on publicly available disclosures, Sprout Health represents one platform operating in this category that provides detailed information across these factors.
Considerations for Anyone Evaluating GLP-1 Telehealth Access:
Compounded medications are not FDA-approved: Compounded drugs are not FDA-approved, and FDA does not review them for safety, effectiveness, or quality before they are marketed.
Prescription is not guaranteed: Independent clinicians determine appropriateness based on individual health factors.
Results vary significantly: Clinical trial results for branded medications do not predict individual outcomes with compounded versions.
Ongoing cost commitment: Benefits typically require sustained use over months, representing significant financial investment.
Regulatory landscape: The compounded GLP-1 telehealth industry has been under increased regulatory scrutiny. Consumers should verify that any platform they consider maintains clear, accurate disclosures.
Regulatory Context:
The compounded GLP-1 telehealth industry has experienced increased FDA scrutiny in recent years, particularly regarding marketing claims that could mislead consumers about the nature of compounded versus FDA-approved medications. Consumers should review the most current information about any platform’s disclosures and verify that claims align with regulatory guidance before proceeding with GLP-1 programs.
Disclaimers
Medical Disclaimer: This article is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Compounded GLP-1 medications require evaluation by a licensed clinician. If you are currently taking medications, have existing health conditions, are pregnant or nursing, or are considering any major changes to your health regimen, consult your physician before starting any GLP-1 medication. Do not change, adjust, or discontinue any medications without your physician’s guidance.
Compounded Medication Notice: Compounded semaglutide and tirzepatide are prescription medications prepared by licensed pharmacies based on individual prescriptions. According to FDA guidance, compounded medications are not FDA-approved as finished products. The FDA does not review them for safety, effectiveness, or quality before they are marketed. They are prepared using active pharmaceutical ingredients under federal and state compounding rules based on prescriptions from licensed clinicians.
Results May Vary: Individual results vary based on factors including age, baseline weight, adherence to treatment, lifestyle modifications, and other individual variables. Clinical trial results cited in this article reflect outcomes from studies of FDA-approved branded medications under controlled conditions and do not predict individual outcomes with compounded formulations.
FTC Affiliate Disclosure: This article contains affiliate links. If you purchase through these links, a commission may be earned at no additional cost to you. This compensation does not influence the accuracy or integrity of the information presented.
Pricing Disclaimer: All prices, subscription terms, and promotional offers mentioned were based on publicly available platform disclosures as of December 2025 but are subject to change. Always verify current terms directly with the platform.
Copyright © 2025. All rights reserved. This article was published December 2025 and reflects information current as of that date.
CONTACT: Email: help@joinsprouthealth.com
Phone: +1 (833) 496-4020


















.jpg)
.jpg)

