VENT-02 demonstrated robust pharmacodynamic modulation, a broad therapeutic index, and potential for once-daily dosing
VENT-02 provided full target coverage with a single dose
No dose-limiting toxicities or serious adverse events observed
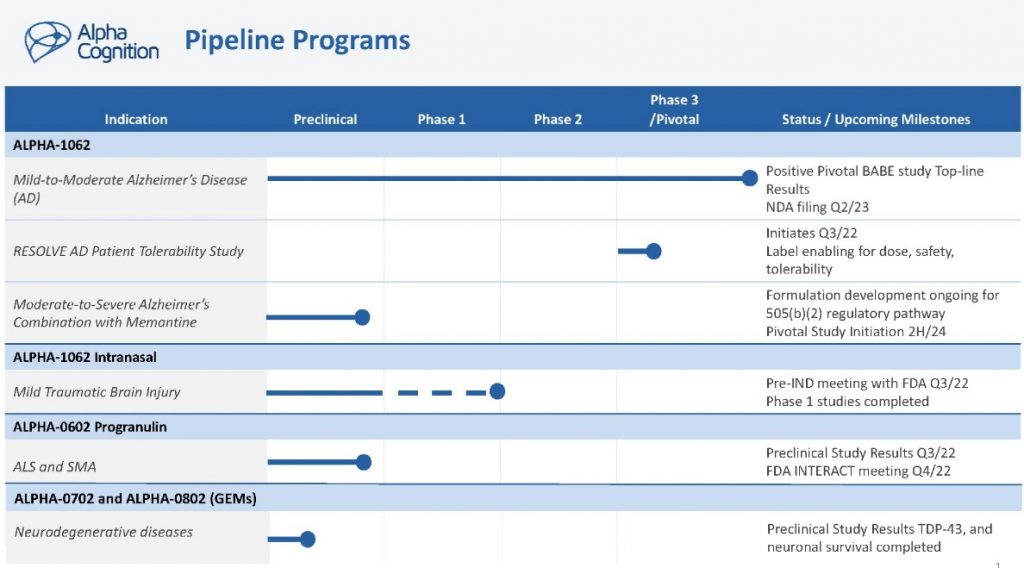
Initiation of clinical trials in CNS diseases expected beginning in the second half of 2024
WALTHAM, Mass. & MONTREAL–(BUSINESS WIRE)–Ventus Therapeutics, a clinical-stage biopharmaceutical company utilizing its proprietary structural biology and computational chemistry platform, ReSOLVE™, to develop differentiated small molecule therapeutics, today announced results from its Phase 1 clinical trial of VENT-02, a novel, oral, brain-penetrant NLRP3 inhibitor. The Phase 1 trial evaluated the pharmacodynamics, pharmacokinetics, safety, and tolerability of VENT-02 across a broad range of single and multiple ascending doses in adult healthy volunteers.

“We are pleased with the results of this trial in which VENT-02 demonstrated a broad therapeutic index and complete target coverage, underscoring its potential to treat diseases of the CNS as well as peripheral diseases with high unmet needs,” said Xavier Valencia, M.D., Head of Clinical Development at Ventus. “Our next trial for VENT-02, a Phase 1b trial in patients with Parkinson’s disease, will evaluate multiple doses and dose regimens against a placebo control and assess a collection of markers that map multiple components of inflammation to disease activity. Based on the promising pharmacodynamic and safety data from this Phase 1 trial and our clinical development plans, we believe VENT-02 is poised to demonstrate best-in-class potential.”
In this Phase 1 clinical trial, VENT-02 was well-tolerated, with no dose-limiting toxicities or serious adverse events reported and only mild or moderate treatment-related adverse events observed. The moderate adverse events included headache and nausea and were only observed at a dose multiple times higher than the intended therapeutic doses.
VENT-02 demonstrated full target engagement through 100% inhibition of IL-1ß in the blood in an ex vivo whole blood challenge assay, significant drug levels in the CSF for 24 hours, and robust reduction of inflammatory biomarkers such as hsCRP. Based on the half-life and target coverage observed in the trial, VENT-02 has demonstrated the potential for once-daily dosing.
“The excellent properties of VENT-02 are a reflection of Ventus’ industry-leading exploration of NLRP3, including demonstrating proof-of-concept in preclinical studies in multiple disease-relevant models, validating biomarkers, and collaborating with academic institutions and the Michael J. Fox Foundation,” said Ventus President and CEO, Marcelo Bigal, M.D., Ph.D. “As a result, we are ready to bring VENT-02 into Parkinson’s disease and other pre-defined diseases where the CNS plays an important role, capitalizing on innovative trials that we are crafting to be informative in reducing risk in the future steps of clinical development. In addition, we expect that our planned trials will allow us to explore the potential impact of NLRP3 inhibition across multiple patient populations suffering from serious diseases.”
Ventus expects to initiate a Phase 1b trial of VENT-02 in patients with Parkinson’s disease in the second half of 2024 and a Phase 2 trial in patients with treatment-refractory epilepsy in 2025.
About NLRP3
NLRP3 is the best understood member of a family of proteins known as inflammasomes. Inflammasomes are multiprotein complexes that regulate the innate immune system and are involved in intracellular surveillance of danger and pathogen signals that trigger an intense inflammatory response, including the release of IL-1β and IL-18 and the induction of pyroptosis, an inflammatory form of cell death. Therapeutic inhibition of NLRP3 can prevent the formation of the NLRP3 inflammasome, which in turn inhibits the production of IL-1β and IL-18 as well as pyroptosis. Aberrant activation of the NLRP3 inflammasome has been associated with systemic conditions, including fibrotic, dermatological, and rheumatological diseases, and is a key driver of disease pathology in several neurological disorders, including Parkinson’s disease, Alzheimer’s disease, and treatment-refractory epilepsy.
About Ventus Therapeutics
Ventus Therapeutics is a clinical-stage biopharmaceutical company deploying deep protein science expertise and a proprietary computational chemistry platform to develop novel small molecule therapeutics for immunology, inflammation, and neurology disorders. Using its proprietary drug discovery platform, ReSOLVE™, the company screened its first target in 2020, selected three development candidates in 2022, and advanced its two wholly-owned product candidates into the clinic in 2023: VENT-03, a potent, selective, oral cGAS inhibitor in Phase 1, and VENT-02, a potent, brain-penetrant, oral NLRP3 inhibitor in Phase 1. In addition, Ventus has out-licensed VENT-01, a peripherally-restricted NLRP3 inhibitor, to Novo Nordisk A/S. These programs exemplify Ventus’ robust discovery capabilities and focus on differentiated programs for multi-indication immunology and neurology targets. For more information, please visit www.ventustx.com and engage with Ventus on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements. These statements involve known and unknown risks, uncertainties, and other important factors that may cause Ventus’ actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “would,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this press release are forward-looking statements, including statements regarding the timing, progress, and results of Ventus’ clinical trials, the anticipated timing of initiation and completion of trials, the period during which the results of the trials will become available, and Ventus’ development plans. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond Ventus’ control, you should not rely on these forward-looking statements as predictions of future events. Such risks include, but are not limited to, the risk that Ventus is unable to further advance VENT-02 and VENT-03 in clinical development, obtain regulatory approval, and ultimately commercialize VENT-02 and VENT-03, or experience significant delays in doing so; that Ventus will require substantial additional funding to finance its operations; that the development and commercialization of pharmaceutical products is subject to extensive regulation, and the regulatory approval processes of the U.S. Food and Drug Administration and comparable foreign authorities are lengthy, time-consuming, and inherently unpredictable; and that Ventus’ success depends on its, its current and future licensors’, and its exclusive licensees’ ability to obtain, maintain, enforce, and protect its intellectual property and its proprietary technologies. Forward-looking statements included herein are based upon information available to Ventus as of the date of this press release, and while Ventus believes such information forms a reasonable basis for such statements, such information may be limited or incomplete, and Ventus’ statements should not be read to indicate that it has conducted an exhaustive inquiry into, or review of, all potentially available relevant information. The events and circumstances reflected in the forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, Ventus does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances, or otherwise.
Contacts
Media
Dan Budwick
1AB
dan@1abmedia.com