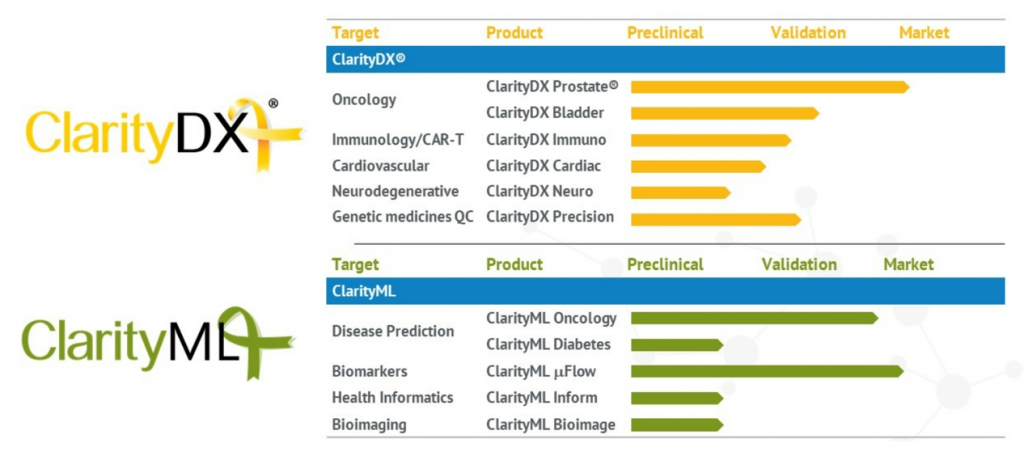
ARMGO Pharma is a privately held biopharmaceutical company dedicated to applying original, targeted science to the discovery and development of novel small-molecule therapeutics to treat debilitating cardiac, musculoskeletal, and neurological disorders. The company’s proprietary drugs, known as Rycals®, are a new class of oral agents that repair calcium leaks through the Ryanodine Receptor (RyR).

ARM210 is a potential disease modifying therapy for genetic diseases, which targets the Ryanodine Receptor calcium channel (RyR), an intracellular calcium-release channel that becomes leaky in these and other diseases. Intracellular calcium leaks via mutant RyR1 channels impair muscle contraction leading to muscle weakness and loss of function, and activate toxic pathways that damage muscle, causing the symptoms in RYR1-RM.
ARM210 is a small molecule that binds to leaky RyR channels and repairs the leak, as demonstrated in vitro in muscle biopsies from RYR1-RM patients. The unique mechanism of action of ARM210 makes it an ideal potential disease modifying therapy for RYR1-RM. Muscle biopsies of the patients in this trial have been previously shown to respond biochemically to ARM210 in vitro. This trial will evaluate the safety and explore the biochemical effect of oral administration of ARM210 in these same patients.
ARMGO Pharma has been awarded an exclusive, worldwide license from Columbia University for its RyR technology based on the research of founding scientist Andrew R. Marks, M.D. In December 2021, ARMGO Pharma raises a $35 million Series B funding round to progress clinical studies of lead molecule ARM210 in cardiac and skeletal muscle diseases.
ARMGO Pharma – developing small molecule Ryanodine Receptor modulators for treating cardiac, musculoskeletal and neurological disorders
More info : www.armgo.com
Keywords : ARMGO Pharma , small molecule , therapeutics , RyR technology , ARM210 , cardiovascular , musculoskeletal disorders , neurological disorders , CNS , neuroscience , Rycals , Ryanodine Receptor , RyR , Ryanodine Receptor modulators