Autophagy is a catabolic process which degrades a cellular own component through the lysosomal machinery. The lysosome, at the heart of the autophagy sytem is important in various processes (cancer, infection…).
In Cancer, they are required in tumor cells for cellular adhesion, motility and signaling, exocytosis, angiogenesis and overall survival, growth, aggressiveness, metastatic potential and drug resistance. Because of their high metabolic rates, rapidly dividing and invasive cancer cells require increased new biomass production to survive. The lysosome is also important for adaptation to nutrient stress as it contains hydrolytic enzymes that play a major role in the degradation of intracellular macromolecules and catabolic (such as autophagy) and anabolic growth. This busy lysosomal behavior leads to alterations in lysosomal structure and function, which, paradoxically, renders cancer cells more sensitive to lysosomal destabilization. In addition, lysosomal enzyme activity is elevated in many tumors compared to adjacent normal tissue, and several reports suggest that lysosomes in tumor cells are more fragile than normal lysosomes. Therefore, lysosome seems to be a target of interest in the fight against cancers. Targeting lysosomes triggers apoptotic and lysosomal cell death pathways.
Genoscience Pharma – new approaches disrupting cancer cell lysosomal functions
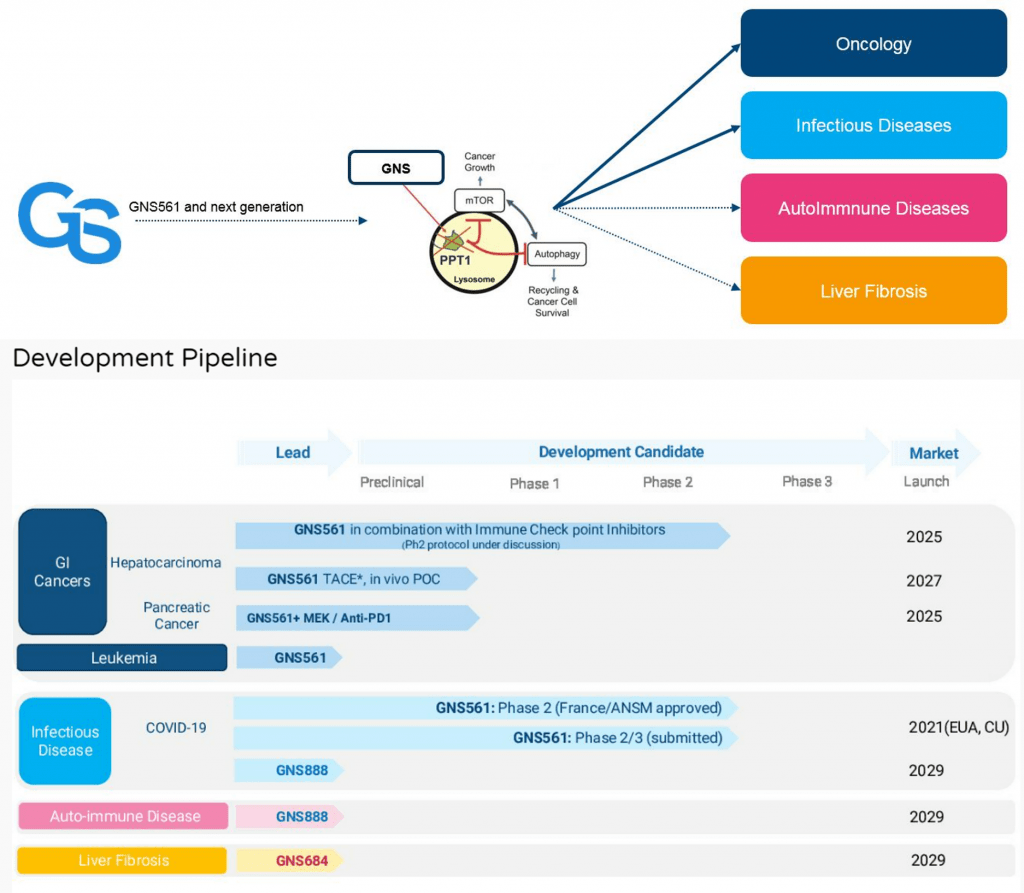
In virology, It has been shown that autophagy is activated during virus and bacterial infection and that some viruses can use the autophagy system to facilitate their own replication . Some viruses, such as Coronaviruses are single stranded, positive sense RNA viruses, which induce the rearrangement of cellular membranes upon infection of a host cell. This provides the virus with a platform for the assembly of viral replication complexes, improving efficiency of RNA synthesis. Genoscience Pharma focuses on new approaches disrupting cancer cell lysosomal functions.
Genoscience Pharma was founded in 2001 by Pr Philippe Halfon, a world renowned medical expert on viral diseases, especially on Human Immunodeficient Virus (HIV) and Hepatitis C Virus (HCV). The company was initially focused on the development of anti-HCV agents. Two protease inhibitors were thus developed and out-licensed to BioLineRX. A new scientific direction was taken in 2012 after the discovery of a new chemical family: autophagy inhibitors. Following promising preliminary in vitro and in vivo results from these small molecules, including against cancer stem cells, Genoscience Pharma has decided to take the opportunity to make the difference in Oncology, especially in cancers where medical needs are still unmet. Now, Genoscience Pharma is a clinical stage biopharmaceutical company focused on translating novel scientific insights into medicines for patients with cancer.
Genoscience Pharma – new approaches disrupting cancer cell lysosomal functions
More info: https://www.genosciencepharma.com/
Keywords : lysosomal functions , cancer, oncology , Genoscience Pharma , lysosomes , infectious diseases , virus , Covid19, leukemia , pancreatic cancer , hepatocarcinoma , small molecules