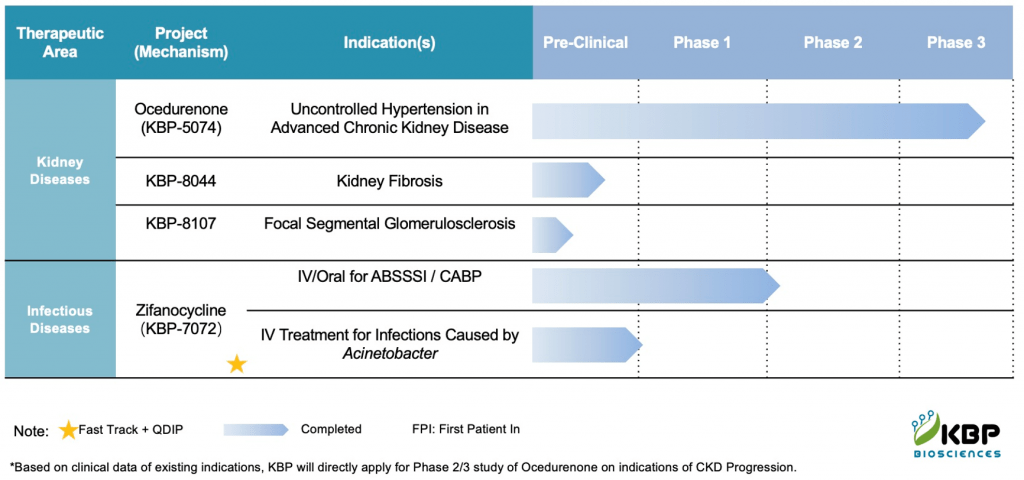
Developed to provide protection for millions of immunocompromised and elderly individuals who are most vulnerable to COVID-19
GHENT, Belgium, June 04, 2024 (GLOBE NEWSWIRE) — ExeVir Bio, a biotech company developing robust heavy chain-only antibody therapies for broad protection against infectious diseases, today announced new data demonstrating that its antibodies are exceptionally potent in neutralizing the SARS-CoV-2 variant JN.1, the parental strain of the currently most dominantly circulating variants worldwide.
ExeVir’s COVID-19 program focuses on addressing the high unmet medical need to protect and treat immunocompromised and elderly individuals that account for respectively 4% and 10% of the global population. Despite the availability of effective vaccines, the immunocompromised and elderly remain at a higher risk for severe COVID-19 as they are often not able to elicit an adequate immune response to vaccination and therefore would strongly benefit from additional protection in the form of antibody therapeutics.
ExeVir’s variant-proof lead clinical candidate XVR013m is a heavy chain-only antibody that targets a unique membrane-proximal epitope in the S2 subunit of the spike protein. This binding region remains completely unmutated across all variants of concern, variants of interest and variants under monitoring that have circulated to date. XVR013m demonstrates exceptional neutralization potency against JN.1, the parental strain of the currently most dominantly circulating variants worldwide, with an IC50 of 2.8 ng/mL in a pseudovirus neutralization assay, unchanged compared to all previously tested variants. The activity against new circulating variants under monitoring is continually being tested.
ExeVir is currently gearing up to start the clinical development of the variant-proof XVR013m in 2025 as a solution for the prevention of COVID-19 in the target populations of immunocompromised and elderly individuals.
Jeanne Bolger, interim CEO of ExeVir, said: “COVID-19 is here to stay and is still the most important respiratory infectious disease causing substantially more hospitalizations and deaths compared to influenza and RSV. Today, no real seasonality is observed for COVID-19, in sharp contrast to influenza and RSV. Our antibody XVR013m is differentiated by targeting a unique and highly conserved epitope in the S2 subunit of the SARS-CoV-2 spike protein that makes it virtually variant-proof.”
Viki Bockstal, CSO of ExeVir, said: “XVR013m not only demonstrates exceptional virus neutralization potency against all variants tested to date, it is also anticipated to maintain its activity against future variants because of the unique nature of the epitope. There is still a strong and urgent medical need for novel antibodies that work against both the newest as well as future SARS-CoV-2 variants, and these new data again demonstrate that XVR013m has the potential to become a durable therapeutic solution to protect the most vulnerable patients from this serious infectious disease.”
About ExeVir Bio
ExeVir Bio is a clinical stage biotechnology company developing heavy-chain only antibody therapies focusing on infectious diseases. The company is harnessing its llama-derived antibody (VHH) technology platform to generate multi-specific antibodies for prophylaxis and treatment of infectious diseases. ExeVir’s initial focus is on prevention of COVID-19 for the immunocompromised patient population, including active chemotherapy, immunosuppressive drugs, solid organ transplantation, hematological malignancies, AIDS patients, and for the elderly, where there remains a high unmet need due to the limitations of current vaccines and therapeutic approaches.
ExeVir has demonstrated it can progress its candidates from research to the clinic in under one year, execute on Phase 1a and Phase 1b studies, and conduct scale-up manufacturing. Leveraging this extensive experience, its XVR013m asset is being developed to start a First in Human clinical trial in 2025.
VHHs are smaller in size than whole antibodies, giving them access to hidden epitopes that traditional monoclonal antibodies are unable to reach with potential for deeper tissue penetration and simpler, more cost-effective manufacturing. VHHs can be linked together like building blocks into single multispecific molecules to tackle different epitopes or act through different mechanisms of actions at once, to address the “arms race” in more complex and co-evolving infectious diseases.
ExeVir is a spin out of VIB, the leading Belgium-based life sciences research institute. It is backed by strong investors including Fund+, which led the series A of EUR 42 million, together with an international consortium including UCB Ventures, SFPIM, V-Bio Ventures, VIB, Wallonie Entreprendre, Noshaq, Vives IUF and SambrInvest. ExeVir has received support from VLAIO, the SPW-Recherche and the European Union, leading to a total of EUR 18 million in non-dilutive funding. In 2023, ExeVir secured an option for EUR 25 million venture debt financing from the European Investment Bank.
References
1. Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study, Evans et al, The Lancet, 2023.
https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762%2823%2900166-7/fulltext.
2. World population above 65 years old: Population ages 65 and above, total | Data (worldbank.org)
For more information contact:
ExeVir Bio
Veronique Vandevoorde
vvandevoorde@exevir.com
Scius Communications (for media)
Katja Stout
+44 778 943 5990
katja@sciuscommunications.com
Daniel Gooch
+44 774 787 5479
daniel@sciuscommunications.com
Find out more on ExeVir’s LinkedIn or on ExeVir’s website