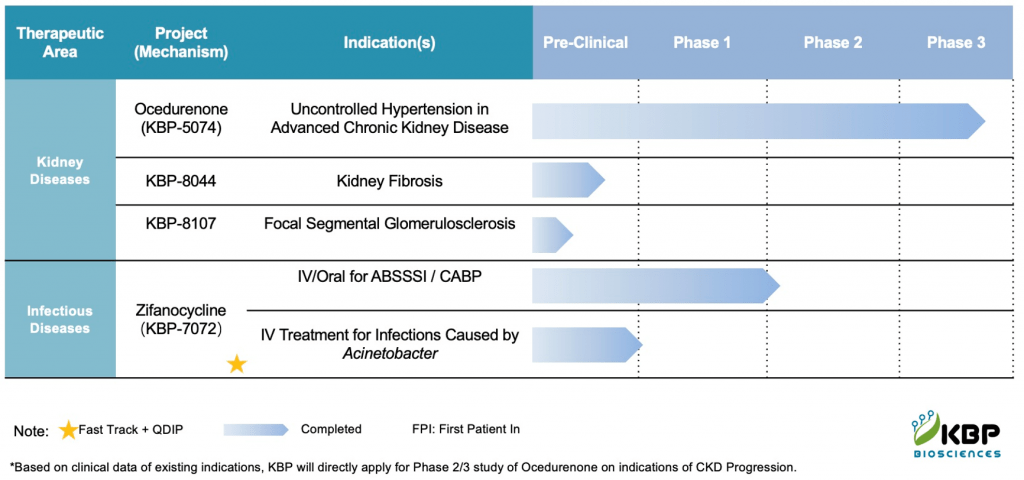
- The Extroducer® Infusion Catheter System® enables local delivery of XC001 to the heart without the need for surgery.
- XC001 has achieved positive Phase 1/2 results in the EXACT Trial validating its transformative potential for treatment of refractory angina in patients who have exhausted available treatment options and have a debilitating quality-of-life.
XyloCor Therapeutics, Inc. (“XyloCor”), a clinical-stage biopharmaceutical company developing novel gene therapies for cardiovascular disease, and SmartWise, a unit of SmartCella Holding AB (“SmartCella”), have entered into a licensing agreement under which XyloCor has rights to the Extroducer® Infusion Catheter System ®, a first-in-class endovascular device designed to deliver advanced therapies directly into the heart [and hard-to-reach tissues] . XyloCor plans to deploy the Extroducer to support catheter-based endocardial delivery of its lead gene therapy candidate, XC001 (encoberminogene rezmadenovec), in future clinical studies and commercial use.
XyloCor and SmartCella enter into license agreement for use of the Extroducer to administer novel gene therapy XC001 to the heart

“This agreement with SmartCella will enable XyloCor to build upon its robust foundation of efficacy and safety data for XC001 by offering the potential for improved safety and ease of delivery without surgery via this novel catheter,” said Al Gianchetti, President and CEO of XyloCor. “Teaming up with SmartCella will help in our effort to optimize patient safety and tolerability while maintaining accurate delivery of XC001 to target areas in the heart for patients with refractory angina. It also opens up the potential to develop XC001 earlier in the coronary artery disease progression for even larger patient groups.”
XC001 is designed to reduce ischemic burden by creating new blood vessels in the heart through the local expression of multiple isoforms of vascular endothelial growth factor (VEGF). With the use of the Extroducer catheter, XyloCor can offer patients a better delivery option for local administration of XC001 directly to the heart, that is less invasive and eliminates potential risks associated with surgical administration.
“We welcome the Extroducer delivery of XC001 as it offers a more efficient method for gene therapy administration for patients with refractory angina,” said Timothy D. Henry MD, Interventional Cardiologist and Director of the Lindner Center, The Christ Hospital, Cincinnati, Ohio. “Preclinical models provide strong evidence that this approach will maintain, or even improve the efficacy when compared to surgical delivery and it should lower the risk of complications that may arise from surgical administration. I am looking forward to initiating the Phase 2b trial of XC001 in patients with refractory angina using this innovative administration approach.”
The recently published EXACT Phase 1/2 trial assessed the use of one-time gene therapy with XC001 as a new therapeutic approach in refractory angina – a debilitating and chronic condition that impacts over one million people in the United States and is growing in prevalence. In the EXACT trial, 42 patients with class II-IV angina were treated with XC001 directly administered to the heart following minimally invasive surgical access. The results demonstrated that treatment with XC001 can be safely administered and achieve durable clinical improvements of exercise duration, and angina frequency, due to a decrease in ischemic burden, as measured by Positron Emission Tomography (PET) imaging. Notably, six months after treatment 43% of patients had no chest pain with ordinary activities and 58% reported no angina episodes at 12-month clinical follow up. XC001 was well tolerated in the patient population and there were no serious adverse events related to the drug. The Phase 2b trial will be a randomized double-blinded study assessing the safety and efficacy of XC001 administered via the Extroducer® Delivery Catheter in coronary artery disease patients with refractory angina.
“The collaboration underscores the transformative potential of the Extroducer in delivering XC001 therapy for patients with refractory angina. A great example of a powerful combination of delivery system and drug therapy representing a substantial advancement in treatment options. The collaboration with such a distinguished partner as XyloCor marks a significant milestone for our global expansion efforts and will also enable us to further explore and harness the future capabilities of the Extroducer, ultimately expanding the benefits to a greater number of patients in need,” said Niklas Prager, CEO of SmartCella.
Terms of the agreement include a global license to XyloCor for use of the Extroducer for the administration of XC001 and provide for SmartCella to supply catheters to XyloCor in clinical trials and commercial use in exchange for an upfront payment, clinical, regulatory and commercial milestones and a royalty on sales. Total deal value amounts to approximately USD 130 million and mid-single digit royalties.
About XC001
XC001 is designed to promote new blood vessels in the heart that will bypass diseased blood vessels and improve blood flow. By restoring blood flow, chest pain associated with refractory angina may decrease, potentially improving patients’ quality of life by enabling them to engage in daily physical activities that would otherwise cause pain. XC001 is designed to avoid toxicity issues observed with other gene therapies through a strategy of one-time, local administration. This approach allows XC001 to achieve higher gene expression in the heart while minimizing systemic vector circulation and associated side effects.
About Extroducer® Infusion Catheter System
The Extroducer® Infusion Catheter System is a first-in-class endovascular delivery device which enables direct-to-tissue drug delivery. The Extroducer® addresses a significant unmet need in the field of novel therapies, enabling targeted delivery of a wide range of modalities for solid tumor treatment, genetic disorders and tissue repair, to name but a few. Using standard equipment and routine interventional radiology approaches, the Extroducer provides access to hard-to-reach tissues by safely penetrating the vessel wall and delivering payload directly to the target location. Smartwise received U.S. Food and Drug Administration (FDA) clearance under 510(k) for the Extroducer® delivery catheter in June 2022.
About XyloCor
XyloCor Therapeutics, Inc. is a private, clinical-stage biopharmaceutical company developing potential best-in-class gene therapies to transform outcomes for patients with cardiovascular disease. The Company’s lead product candidate, XC001, is in clinical development to investigate use for patients with refractory angina for whom there are no treatment options. XyloCor has a second preclinical investigational product, XC002, in discovery stage, being developed for the treatment of patients with cardiac tissue damage from heart attacks. The company, which was co-founded by Ronald Crystal, M.D., and Todd Rosengart, M.D., has an exclusive license from Cornell University. For more information, visit www.xylocor.com.
About SmartCella
SmartCella, founded in 2014, is an innovative biotechnology company based in Stockholm, Sweden. SmartCella’s vision is to combine first-in-class delivery platforms with cutting-edge cell and mRNA therapies to unleash the full potential of targeted therapies. The company has three main business units, Smartwise, SmartCella Solutions and ProCella. For more information, visit www.smartcella.com.