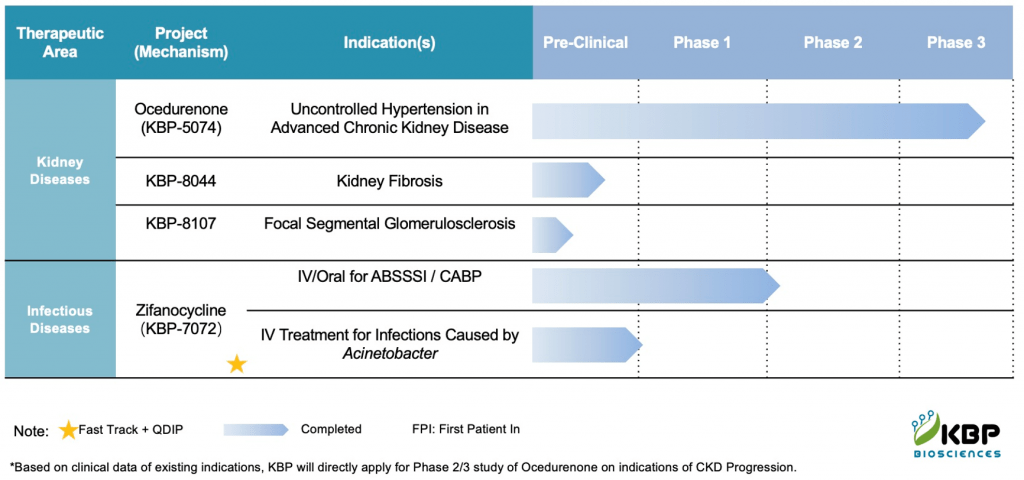
Green Light for Phase 2 Trial of AST-004 in Acute Ischemic Stroke Patients
GROTON, Conn., March 12, 2024 (GLOBE NEWSWIRE) — Astrocyte Pharmaceuticals Inc., a clinical-stage biopharmaceutical company committed to advancing cerebroprotective therapies for individuals suffering from stroke, traumatic brain injury (TBI), and concussion, has announced today the U.S. Food and Drug Administration (FDA) has cleared the company’s Investigational New Drug (IND) application for its lead program, AST-004, for acute ischemic stroke. The company is preparing to initiate a Phase 2 clinical trial. The IND application clearance also paves the way for additional studies further exploring AST-004 in stroke, as well as TBI, and concussion.
Currently, only ~5% of stroke patients receive pharmaceutical therapy, largely due to the short treatment windows of available thrombolytic medicines, as well as their need for imaging before those treatments can be initiated. AST-004 was developed with the potential to treat all stroke patients. Unlike existing therapies, treatment with AST-004 is designed for use without the need for imaging in the emergency room and for use at any point after diagnosis. And it has the potential to treat stroke occurring in any sized blood vessel in the brain.
The upcoming Phase 2 trial in acute ischemic stroke patients builds upon the recent successful stroke clinical trial designs used by medical device companies, heralding a new era in patient care. “The past decade has witnessed a transformative shift in stroke trials, thanks to sophisticated imaging techniques that enable high-quality real-time characterization of stroke and ensure selection of homogeneous study groups,” said Dr. Kevin Sheth, Co-Director of the Center for Brain & Mind Health at the Yale School of Medicine and Chief Medical Advisor to Astrocyte. “The new study of AST-004 will first focus on treating those stroke patients with large vessel occlusions, offering hope to significantly diminish brain damage.”
AST-004 is an innovative small molecule therapy that has demonstrated exceptional promise in preclinical studies. In a critical non-human primate stroke model, a significant reduction in brain lesion growth by 64% and an overall lesion size decrease by 45% were observed with a high dose (as reported in ‘The Journal Stroke’ in 2022). Astrocyte previously completed two Phase 1 safety trials in Europe, involving 80 participants in total, and saw no significant adverse effects or safety concerns.
Dr. William Korinek, CEO of Astrocyte Pharmaceuticals, commented, “Since our founding in 2014, we have been focused on demonstrating the potential of cerebroprotective benefits of AST-004. Our robust preclinical program and Phase 1 studies have laid the foundation for our ongoing development efforts, and we are eager to move forward with the planned Phase 2 study. Stroke is the second leading cause of death globally, and the need for advances in treatment is enormous. We believe AST-004 can be part of that solution.”
About Astrocyte Pharmaceuticals Inc.
Astrocyte Pharmaceuticals Inc. is a privately held, clinical-stage drug discovery and development company dedicated to accelerating the recovery and well-being of brain injury patients. The company is committed to proving the cerebroprotective benefits of enhancing astrocyte function and advancing breakthrough therapeutic agents for treating brain injury resulting from stroke, traumatic brain injury, concussion, and neurodegenerative disorders such as Alzheimer’s disease. For more information on Astrocyte Pharmaceuticals Inc. and the AST-004 program, please visit us at Astrocyte Pharmaceuticals Inc.
Forward-Looking Statement
This press release contains certain forward-looking statements regarding, among other things, statements relating to goals, plans, and projections regarding the company’s financial position, results of operations, market position, product development, and business strategy. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert, or change any of them and could cause actual outcomes and results to differ materially from current expectations. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. The information in this release is provided only as of the date of this release, and the company undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
Media contact:
Kimberly Macleod – kim@kmacconnect.com
info@astrocytepharma.com